For Which H-atom Wavefunction Are You Most Likely To Find The Electron Farthest From The Nucleus?
Learning Objective
- Distinguish betwixt electron orbitals in the Bohr model versus the quantum mechanical orbitals
Key Points
- The Bohr model of the atom does non accurately reflect how electrons are spatially distributed around the nucleus as they do non circle the nucleus like the globe orbits the sun.
- The electron orbitals are the effect of mathematical equations from quantum mechanics known as wave functions and tin predict within a certain level of probability where an electron might be at whatsoever given time.
- The number and type of orbitals increases with increasing atomic number, filling in various electron shells.
- The area where an electron is most likely to be found is chosen its orbital.
Terms
- electron shellThe collective states of all electrons in an atom having the same primary breakthrough number (visualized every bit an orbit in which the electrons motility).
- orbitalA specification of the free energy and probability density of an electron at any point in an atom or molecule.
Although useful to explain the reactivity and chemical bonding of certain elements, the Bohr model of the cantlet does not accurately reflect how electrons are spatially distributed surrounding the nucleus. They practise not circle the nucleus like the earth orbits the sun, merely are rather found in electron orbitals. These relatively complex shapes result from the fact that electrons behave not just like particles, but also like waves. Mathematical equations from quantum mechanics known equally wave functions can predict inside a certain level of probability where an electron might exist at whatsoever given time. The surface area where an electron is most likely to be institute is called its orbital.
Outset Electron Shell
The closest orbital to the nucleus, chosen the 1s orbital, can concur up to two electrons. This orbital is equivalent to the innermost electron crush of the Bohr model of the cantlet. It is called the 1s orbital because it is spherical around the nucleus. The 1s orbital is always filled before whatever other orbital. Hydrogen has 1 electron; therefore, it has only i spot within the 1s orbital occupied. This is designated every bit 1sane, where the superscripted ane refers to the 1 electron within the 1s orbital. Helium has ii electrons; therefore, it tin can completely fill up the 1s orbital with its two electrons. This is designated as 1s2, referring to the two electrons of helium in the 1s orbital. On the periodic tabular array, hydrogen and helium are the simply ii elements in the offset row (period); this is because they are the sole elements to have electrons but in their first shell, the 1s orbital.
Second Electron Shell
The second electron shell may contain eight electrons. This crush contains another spherical south orbital and iii "dumbbell" shaped p orbitals, each of which can agree two electrons . After the 1s orbital is filled, the second electron beat is filled, first filling its 2s orbital and then its three p orbitals. When filling the p orbitals, each takes a single electron; in one case each p orbital has an electron, a second may be added. Lithium (Li) contains three electrons that occupy the first and second shells. 2 electrons fill the 1s orbital, and the tertiary electron then fills the 2s orbital. Its electron configuration is 1s22s1. Neon (Ne), on the other hand, has a total of ten electrons: ii are in its innermost 1s orbital, and eight fill its second shell (two each in the 2s and 3 p orbitals). Thus, information technology is an inert gas and energetically stable: it rarely forms a chemic bail with other atoms.
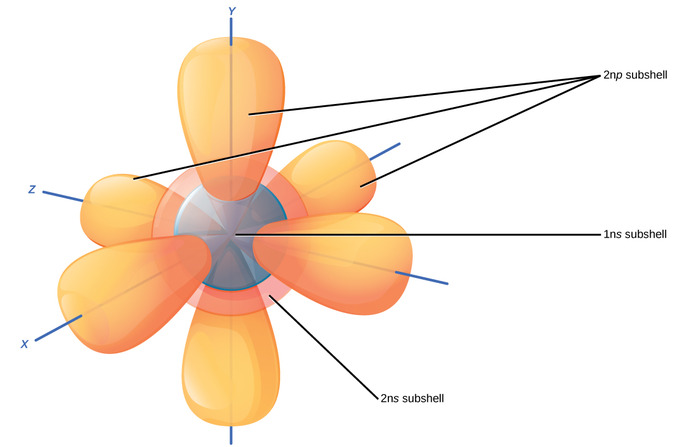
Third Electron Shell
Larger elements have boosted orbitals, making up the 3rd electron vanquish. Subshells d and f have more complex shapes and contain five and 7 orbitals, respectively. Principal crush 3n has s, p, and d subshells and can hold xviii electrons. Principal crush 4n has s, p, d, and f orbitals and can hold 32 electrons. Moving away from the nucleus, the number of electrons and orbitals found in the energy levels increases. Progressing from one atom to the next in the periodic table, the electron construction tin be worked out by fitting an extra electron into the next available orbital. While the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom because the orbital model specifies the different shapes and special orientations of all the places that electrons may occupy.
Bear witness Sources
Licenses and Attributions
Source: https://www.coursehero.com/study-guides/introchem/electron-orbitals/
Posted by: machadopriandn.blogspot.com

0 Response to "For Which H-atom Wavefunction Are You Most Likely To Find The Electron Farthest From The Nucleus?"
Post a Comment